Biologics and biosimilars can help patients with moderate-to-severe inflammatory bowel disease (IBD). You might have questions about how these drugs work. AGA provides answers to frequently asked questions (FAQs) about biologics and biosimilars.
Visit our biosimilars page for an overview that includes how to talk to your provider about biosimilars and who should take them.
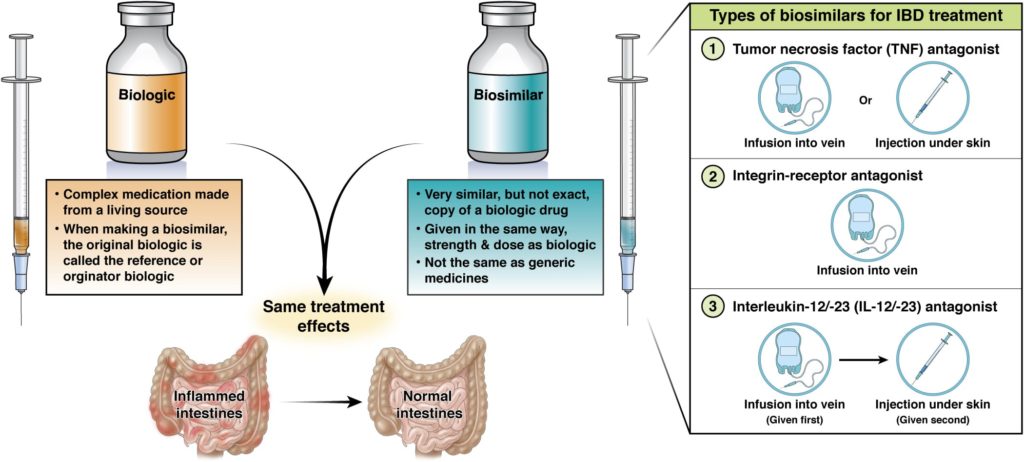
Biologics are a type of drug used to treat disease. Most drugs are small molecules made using simple chemical processes. Biologics, on the other hand, are large and complex antibody proteins derived from living organisms and are manufactured using cutting-edge technology. Because antibodies are highly specific in what they bind to, they can be used as a more targeted treatment than normal medications.
Biosimilars are highly similar to, but not exact copies of, existing biologics.
A biosimilar differs very slightly from the original biologic, only because the way biologics and biosimilars are made is so complex. However, biosimilars are identical in their protein amino acid structure.
The company that makes the biosimilar tests it in people (in clinical trials) to make sure it has no clinically meaningful differences from the original biologic. Biosimilars are given the same way, at the same strength and dose as the original biologic. They have the same treatment benefits, but also the same risks and potential side effects as the original biologic. Injectable biosimilars may have an auto-injector that looks different from the original biologic, but it will function the same.
Biosimilars are not generics, but they are alike in the sense that they: 1) are both versions of “brand name” drugs, 2) may cost less, and 3) become available after the original drug loses its exclusive patent. Biosimilars and generics are also both approved through shorter pathways that avoid duplicating costly clinical trials.
Generics and biosimilars are different in that generics are exact copies of drugs with known chemical structures. Biosimilars—because they are large and complex proteins—may differ slightly from the biologics they are based on (but not in a way that affects their function).
Biosimilars are developed under stricter rules, requiring near-equivalent efficacy to the originator molecule. Generics have less stringent criteria, requiring at least 80% equivalence to the original drug.
The U.S. Food and Drug Administration (FDA) requires many tests to show that a biosimilar is highly similar to the original biologic. Before a biosimilar is made available, it must clear these hurdles:
- Studies must show it works the same as the original biologic—with no clinically meaningful differences in terms of how well it works and how safe it is.
- It must be available in the same forms and doses as the original biologic.
- It can only be used to treat the diseases that the original biologic is used for.
Read more about the FDA process.
If you are starting a biosimilar without ever having tried the original biologic, ask your gastroenterologist about the timeframe they have in mind for looking at how this new treatment is working for you.
If you’re switching from the original biologic to a biosimilar, there’s no reason to think that the biosimilar won’t work the same as the original biologic. But let your health care provider know if you have any concerns.
Interchangeability means that, even though the original biologic and the biosimilar are not exactly the same, either can be used and the same results will be expected. A pharmacist can give a patient an interchangeable drug without asking the health care provider who wrote the prescription if it’s okay. However, different states may have different rules about this.
All approved biosimilar drugs, whether or not they are designated interchangeable, meet the FDA’s high standards.
Research says yes. You should be able to switch from the original biologic to a biosimilar without problems. Your disease signs and symptoms should still be controlled in the same way, and you should not have any new side effects from the biosimilar.
Research suggests that if you do well on the original biologic, you will do well on a biosimilar. If that turns out not to be the case, it could be due to a disease flare or an infection unrelated to the biosimilar. Or, symptoms could be due to something called a “nocebo” effect, which means that if you are anxious that you might do poorly on a drug, that could come to pass. Your health care provider can work with you to figure out if you are having a true flare, if your symptoms are due to some other medical reason, or if concerns about the biosimilar are behind what’s going on.
If a biosimilar is your first biologic drug, your health care provider will measure how well it works the same as they would with any other biologic.
Research says yes. There’s no reason to think they won’t work just as well.
Research says yes. There’s no reason to think they won’t be just as safe.
Yes. Many biosimilars are already available—and more are likely coming.
You may hear a biologic called the “reference” or “originator” biologic or product. That means it was the initial or original (first) biologic drug of its type approved by the FDA. These are the medicines that biosimilars are modeled after.
The frequency of treatment will be the same with the original biologic and the biosimilar.
Research shows there are no drawbacks, but there are likely also no added benefits. Biosimilars may cost less than biologics, but you may not see those savings.
For insurance companies, hospitals, and people paying for their own medicines, there may be savings using a biosimilar instead of the original biologic. People with insurance may not see any difference.
Due to the manufacturing process and how long they take to test and develop, biologics are among the most expensive prescription drugs available. Biosimilars may cost less because manufacturers rely on the FDA’s finding that the original biologics are safe and effective. Biosimilars may be covered by more insurance companies due to lower costs. However, you still may have trouble paying for a prescribed biologic or biosimilar. Many manufacturers offer copayment assistance programs to help you cover the costs of your medications. Ask your providers office for more information or contact the manufacturer directly.
REVIEWED BY

Joseph D. Feuerstein, MD, AGAF
Clinical chief, gastroenterology
Beth Israel Deaconess Medical Center

Sarah Streett, MD, AGAF
Clinical professor, medicine - gastroenterology & hepatology
Stanford Medicine
Updated Nov. 13, 2025