What is a biosimilar?
Biosimilars are a type of biologic. They are based on, and may be used instead of, a reference biologic, which is the name given to the first of a given type of biologic.
The market for biologics, including biosimilars, is expected to grow. This should benefit you and your health care provider by increasing treatment options and improving access to vital drugs at lower costs.
For a biosimilar to be approved by the U.S. Food and Drug Administration (FDA), it must:
- Meet very strict standards to show that it works the same way as the reference biologic and has no meaningful clinical differences in terms of safety and effectiveness.
- Be given the same way, have doses offered in the same form, and have the same strength as the reference biologic.
- Only be used for reasons and conditions for which the reference biologic is approved.
As biosimilars become more available, it is important for you and your health care provider to understand what they are and how they can be used to treat a wide range of diseases.
All FDA-approved biosimilars share the same safety and effectiveness as the originator treatment options and are prescribed the same as the reference biologic.
What is interchangeable?
Some biosimilars have been designated “interchangeable,” which is a specific designation given to certain versions of these products. The FDA states, “An interchangeable biological product is a biosimilar that meets additional requirements and may be substituted for the reference product at the pharmacy, depending on state laws.” This means that if a patient is prescribed a brand biologic by their physician, the pharmacist can automatically dispense the interchangeable biologic in its place without approval from the physician.
For a product to receive an “interchangeable” designation, the manufacturer must submit additional data to the FDA to prove that it can be seamlessly substituted without impacting a patient’s treatment plan. However, all biologics and biosimilars have to meet strict quality and safety regulations.
Biosimilars should not be confused with generic medicines (generics).
- A generic is an exact copy of a chemically formed brand name drug.
- A biosimilar is highly similar with no clinically meaningful differences to, but not an exact copy of, a reference biologic which is already on the market and approved by the FDA.
- Proving that a biosimilar is highly similar with no clinically meaningful differences to its reference biologic is a complex process with many steps including showing the same safety effectiveness.
Who should take a biosimilar?
Biologics, including biosimilars, can help patients with moderate-to-severe inflammatory bowel disease (IBD) who have not responded to or are not candidates for other treatments. Biologics are an important treatment option for patients with IBD. They work by targeting specific proteins or cells in the immune system (the body’s natural defense against illness and germs) involved in the inflammatory process.
IBD most often refers to two conditions — ulcerative colitis and Crohn’s disease — which are characterized by inflammation (swelling) of different parts of the digestive tract. The exact cause of IBD is complex, but research shows it may be the result of someone’s immune system attacking healthy cells and tissue in the digestive tract.
Although IBD cannot be cured, reference biologics and biosimilars can both help lessen or eliminate symptoms, known as remission, and improve quality of life for many patients with moderate to severe IBD. They also have an advantage over other medicines used in IBD, as they are more targeted and selective in the body and in their medical effect. This means they might be more effective and have less side effects. To see the best results, it’s important to stay on therapy.
Choosing a biosimilar
Biologics, including biosimilars, for IBD work by targeting specific proteins or cells in the immune system involved in the inflammatory process.
Biosimilars are not a compromise. Biosimilars are biologics – same medicine, different brand name. Biosimilars must meet the same high manufacturing standards and are produced in the same way. You can feel confident about your treatment no matter what brand is selected.
The selection of which biologic or one of its biosimilar equivalents is an important decision to make with your health care provider, factoring in how they work and which aspects of IBD they most effectively target. Once the mechanism/therapy is selected, either the originator or its biosimilar equivalent will be dictated by the coverage policy of the insurer.
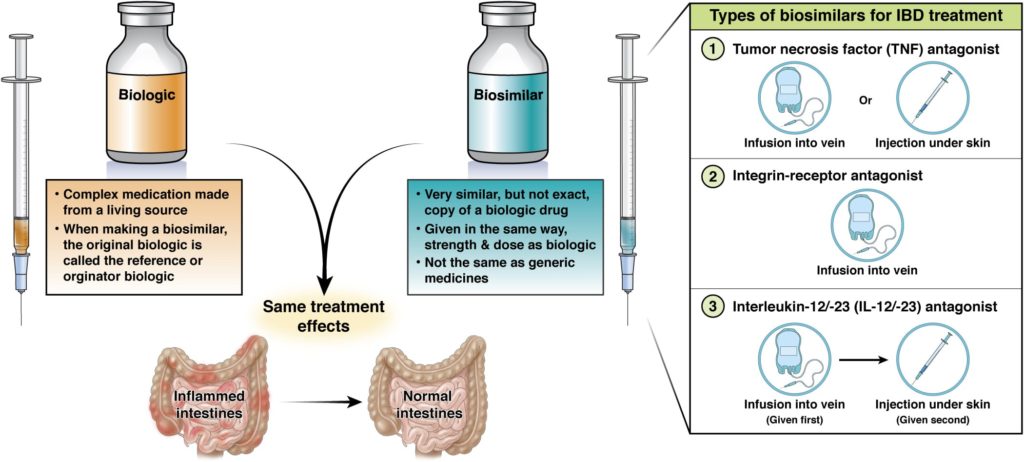
At this time, there are three types of biologics used to treat IBD
-
Tumor necrosis factor (TNF) antagonists
Biologics that block a protein called tumor necrosis factor (TNF) that causes inflammation of the intestine and other body organs or tissues. These are given by injection under the skin or infusion into a vein.
- Infliximab
- Adalimumab
- Golimumab
- Certolizumab
-
Integrin-receptor antagonists
Biologics that block a protein on the surface of immune cells that contribute to inflammation. These are given by infusion into a vein.
- Vedolizumab
-
Interleukin-12/-23 (IL -12/-23) antagonists
Biologics that stop two cytokines (IL-12 and IL-23) from causing inflammation in Crohn’s disease. These are given by infusion into a vein or injection under the skin.
- IL-12/-23-specific therapy
- Ustekinumab
- IL-23-specific-therapies
- Guselkumab (Tremfya®)
- Risankizumab (Skyrizi®)
- Mirikizumab (Omvoh®)
Work with your GI to decide which medicine(s) are right for you
When considering biologics, including biosimilars, as a potential treatment, it is important for you and your health care provider to work closely together and discuss these medicines thoroughly. This will help ensure you have the information you need to take the medicine safely and effectively.
Some key points to discuss include:
- The types of biologics, reference biologics, and biosimilars available and their purpose.
- How the medicines are administered (usually by injection or intravenous [IV] infusion).
- The benefits of taking these medicines (they offer targeted and effective treatment with a low incidence of possible side effects).
- The risks and possible side effects of these medicines (these can be different for each biologic).
There may be times when your insurance company switches from covering a biologic to a biosimilar, requiring patients to change medicines. Usually, this results in cost savings for the insurance company and has the potential to also result in savings for patients, in the form of lower drug costs and co-payments. Switching from a biologic to its biosimilar is safe. Let your health care provider know about any change in your insurance coverage and any questions you have about switching to a biosimilar.
Sticking to your treatment plan
Reference biologics and biosimilars are medicines that need to be taken consistently and as directed by your health care provider.
Not following your health care provider’s plan for how much of your biologic or biosimilar to take, when to take it and how to take it may cause your body to mount an immune response, making the treatment ineffective and increasing the risk for complications, such as allergic reactions.
The body’s production of an antibody [cells that fight disease] that binds to and neutralizes the drug can lead to loss of response. This can occur with biologics and biosimilars, and may require an increase in dose, a change in how often the drug is taken, or a change to another biologic or biosimilar medicine.
Work closely with your health care provider to understand the importance of taking your medicine as prescribed. It is vital to get the best possible results.
Talk with your gastroenterologist and other health care providers about your IBD and your needs. This will help you feel like your needs have been put first, which has been linked to staying on a treatment plan, better adherence to treatments, and better clinical outcomes.
Safety of biosimilars
Both biologic reference products and biosimilars have unique and rigorous pathways for FDA approval. These usually involve additional quality assurance testing compared to chemically-formed drugs, as well as clinical and non-clinical data to ensure safety and effectiveness for patients.
Use of these medicines is carefully watched when they enter the market. You can help improve the safety of these drugs by taking your medicine as directed and reporting any side effects or adverse reactions as soon as they occur.
What do biosimilars cost?
Each insurance company covers reference biologics and biosimilars differently. Before initiating or changing therapy, check your insurance coverage. Manufacturers frequently offer patient-assistance programs to help make treatment more affordable.
If your insurance company requires you to first try and see if another medicine works for you before agreeing to cover a therapy prescribed by your health care provider, work with your provider’s office to contact your state health department to file an appeal.
REVIEWED BY

Joseph D. Feuerstein, MD, AGAF
Clinical chief, gastroenterology
Beth Israel Deaconess Medical Center

Sarah Streett, MD, AGAF
Clinical professor, medicine - gastroenterology & hepatology
Stanford Medicine
Updated June 17, 2025


